Industries
January 2022
Product design and development should aim for a device that allows safe and effective use. This is especially true in the case of the trend toward the increasing fusion of medical devices and consumer goods. For special user groups that cannot be expected to be fully engaged with usage of the device, the aim of safe and effective use is even more pressing. This issue is increasingly becoming more complex and represents a challenge for manufacturers. The interdisciplinary development teams at Helbling have extensive experience in providing product design focusing on special user groups like children, elderly people, or even cats and veterinarians. With a user-centered design approach and the inclusion of psychological theories such as the Fogg behavior model, Helbling is achieving the engagement of non-compliant users.
What does ‘non-compliant user’ mean? Picturing a child lying on the floor of a shopping mall and screaming might be rather extreme, but an image of a person standing with their arms crossed, refusing to use a product gives a good idea of this. Depending on the product and user groups, developers might have to deal with users that are either not willing to or not capable of using a product with high motivation and commitment. Helbling has observed a growing need for support with these topics from clients over recent years. Ease of use is taking a more central role, especially for medical devices. Firstly, these products are increasingly merging with consumer goods for the general population, for example in the form of smart devices and apps. Secondly, there are more and more specialized medical devices for individual home use.
Some users’ circumstances when interacting with a device might affect their compliance, e.g., if in pain, with limited consciousness, or restricted by age. Helbing is currently developing an implant for elderly people that can be adjusted by a remote control, which poses challenges regarding limited user abilities in terms of cognitive and tactile capabilities.
What to do if a user does not want to use a product? Make it invisible.
Designing products for users who might not be thrilled to use the device is a special challenge for the design and development team. For medical devices, it can be about life and death, or at least about successful medical treatment. Apart from ensuring the proper treatment of a patient, it also serves as relief for patients’ caregivers, such as parents or relatives who are supporting treatment.
Therefore, usage of a device should be integrated into daily life and only require very little change to existing routines for patients. Understanding the touchpoints of patients and guardians with the device in daily use and identifying possible pain points is a must. To achieve this, Helbing follows a user-centered design approach and establishes close collaboration with users by interviewing and observing them in context with the relevant use scenarios throughout the entire development process. Contextual inquiries and usability tests also provide an understanding of possible usability risks and hazards that will need to be addressed through the medical device’s design. Helpful design factors can be the shape of the device, colors, sound, and vibrations.
How to hide a product? Integrate it into an existing daily routine.
When analyzing user’s routines, it is important to identify options that will enable integration of a medical device. Helbling’s interdisciplinary development team is currently developing an eye treatment for children by designing special glasses that can be substituted for a child’s existing standard glasses. To generate the best possible solution, technical experts, software developers, industrial designers, and usability experts work closely together.
Even at an early stage in a project, the opportunity should be taken to ask a user to interact with a simple prototype (e.g., paper prototype). Helbling approaches this with an iterative usability process. The first step during user research involves covering general parameters, such as the acceptable size and weight for a pair of glasses. In a following iteration, integration of technical means and how these affect wearer comfort can be assessed in a formative usability test. Eventually, the whole package with accessories and labeling are evaluated. Depending on the product, these usability tests can be set up in different ways. If a product is used indoors, it might be sufficient to invite some potential users to the office and observe their performance. Conversely, if a product should be used outdoors or in specific environments, it might be advisable to simulate the environment or actually go outside if safety concerns allow for this. Since parameters like background noise, distractions, and light conditions can have a severe impact on device handling, these topics should not be neglected.
What to do with the remaining interaction? Make it fun!
Product usage should be designed with ease of use in mind, making handling as simple as possible. Helbling’s usability experts and industrial designers identify user mental models and ability ranges to decide which design approach will be the most beneficial for the user. Depending on the user groups, motivational triggers (sparks) can be added, which should improve user adherence to treatment, as described in the Fogg behavior model. This is particularly of note if successful treatment requires a general change of a daily routine, for example a change of food intake to lose weight, as in a current Helbling project. To bring about a behavioral change in the patient, in addition to easy handling, the product can provide regular motivational triggers and positive reinforcement to keep motivation up. This can be through feedback on the device itself, supportive pop-up messages in an app, or a link to a community of like-minded people. Helbling is involved in an app development supporting overweight users to comply with their treatment. The aim of reducing food intake will be supported by providing sparks and giving access to a support community.
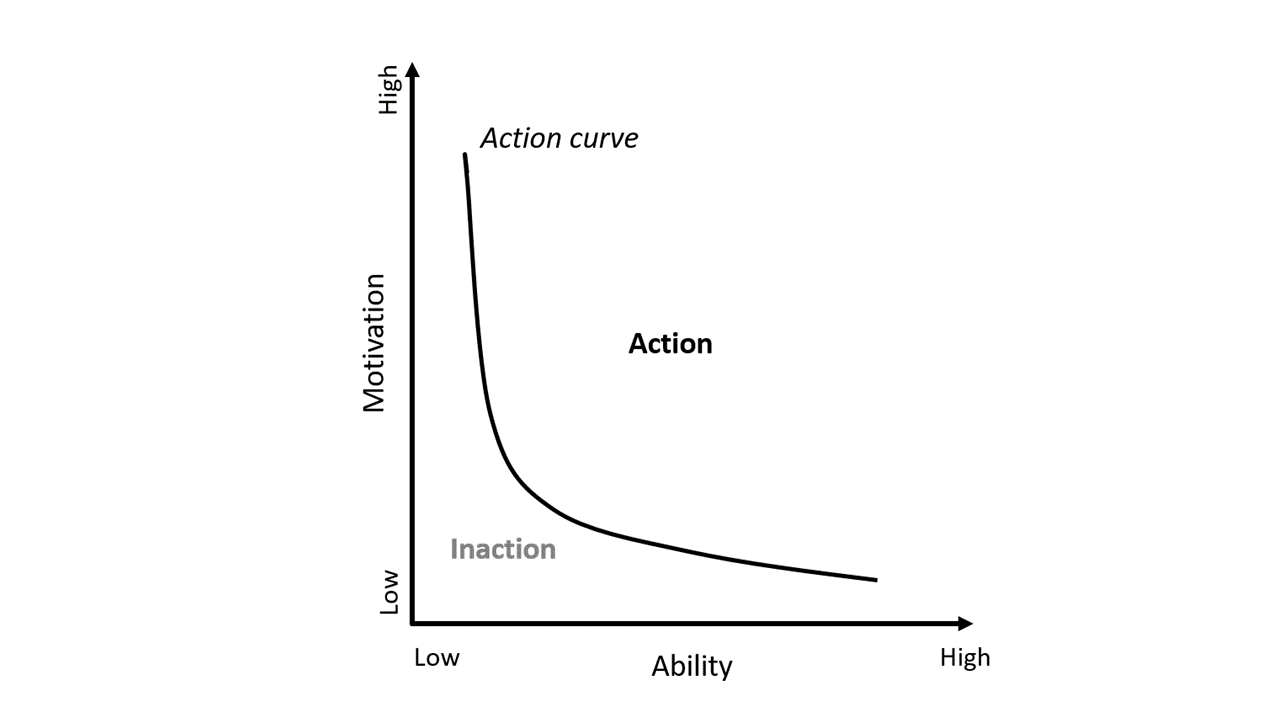
Summary
Focus on user compliance must be a central part of product development.
Especially for medical devices, where high user compliance can be essential for a good medical outcome, special consideration must be given to non-compliant user groups. Assessing their needs and designing a device that requires as little effort to use as possible will improve the chance of safe and effective usage. In addition, supporting a behavioral change toward increased compliance can improve the medical outcome. Achieving this requires close interaction with users in an iterative way throughout the entire development process. Helbling’s iterative user-centered design approach and robust development process ensure the user-friendly, safe design of medical devices and consumable products, also securing the engagement of non-compliant users.
Author: Dorothee Weichel